The human body is a marvel of intricate biological functions, and among its vital components are ISLET cells. These tiny but powerful clusters of cells, found in the pancreas, play a crucial role in maintaining blood sugar levels. For individuals battling diabetes, ISLET cells are of particular interest, as they are directly responsible for insulin production. In this blog, we explore the significance of ISLET cells, their role in diabetes, and the latest advancements in ISLET cell transplantation.
What Are ISLET Cells?
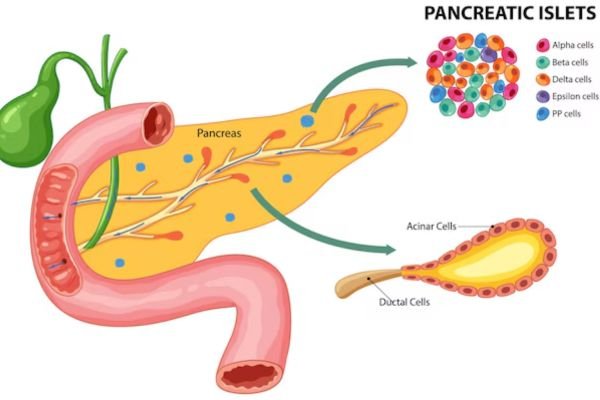
ISLET cells, or pancreatic islets, are clusters of hormone-producing cells within the pancreas. Also known as the Islets of Langerhans, these cell groups are named after Paul Langerhans, the scientist who discovered them in the 19th century. Each pancreas contains about 1 to 2 million islets, making up about 1-2% of the organ’s total mass.
ISLET cells consist of several types of hormone-secreting cells, including:
- Beta Cells: Produce insulin, which lowers blood glucose levels.
- Alpha Cells: Secrete glucagon, which raises blood glucose levels.
- Delta Cells: Release somatostatin, which regulates the other hormones.
- PP Cells: Secrete pancreatic polypeptide, which influences digestion.
Among these, beta cells are the most significant in diabetes management, as they produce insulin, the hormone responsible for regulating glucose levels in the bloodstream.
ISLET Cells and Diabetes
Diabetes, particularly Type 1 diabetes, occurs when the body’s immune system mistakenly attacks and destroys beta cells. This leads to a lack of insulin, resulting in high blood sugar levels. Type 2 diabetes, on the other hand, is characterized by insulin resistance, where the body’s cells do not respond properly to insulin, leading to elevated glucose levels.
When beta cells are damaged or dysfunctional, the body cannot regulate glucose efficiently, leading to serious complications such as heart disease, kidney failure, and nerve damage.
ISLET Cell Transplantation: A Revolutionary Approach
One of the most promising treatments for Type 1 diabetes is ISLET cell transplantation. This procedure involves transferring healthy ISLET cells from a donor pancreas into a diabetic patient. The goal is to restore insulin production and eliminate the need for external insulin injections.
How Does ISLET Cell Transplantation Work?
- Donor Selection: ISLET cells are harvested from a deceased donor’s pancreas.
- Cell Isolation: The ISLET cells are carefully extracted and purified.
- Transplantation: The cells are infused into the liver through a small catheter.
- Engraftment & Functionality: If successful, the transplanted cells start producing insulin naturally, reducing or eliminating the need for insulin therapy.
Challenges and Future of ISLET Transplantation
While ISLET cell transplantation shows promise, there are several challenges, including:
- Immune Rejection: The body may reject the transplanted cells, requiring lifelong immunosuppressive drugs.
- Limited Donor Availability: There is a shortage of donor pancreases for transplantation.
- Cell Longevity: Transplanted ISLET cells may not survive long-term, necessitating repeat procedures.
To overcome these hurdles, researchers are exploring stem cell-derived ISLET cells, which could provide an unlimited supply of insulin-producing cells without the need for donors.
Conclusion
ISLET cells play a vital role in regulating blood sugar levels, making them central to diabetes management and treatment. Advances in ISLET cell transplantation and regenerative medicine are paving the way for potential cures for Type 1 diabetes. As research continues, we may soon witness groundbreaking therapies that restore natural insulin production and improve the lives of millions affected by diabetes.
For those seeking to understand more about diabetes reversal and innovative treatments, stay tuned for further updates on cutting-edge medical breakthroughs!